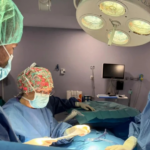
Un trabajo de reconstrucción de la mama recibe el Premio Humanización
9 octubre, 2025
Cancer Research Institute- Clinic and Laboratory Integration Program 2025
9 octubre, 2025Advancing Breakthrough Immunotherapy Trials to Transform Patient Care
The CRI Clinical Innovator supports pioneering immunotherapy clinical trials designed and led by academic researchers. These investigator-initiated studies are vital to improving patient outcomes, addressing areas of high unmet needs and generating critical mechanistic insights than can guide the future of cancer treatment.
The CRI Clinical Innovator funds phase I or II clinical studies with a strong emphasis on:
- Novel immunotherapy approaches
- Mechanistic investigations into clinical response
- The discovery and/or validation of predictive biomarkers
Applications are evaluated based on novelty, feasibility, scientific rationale, and potential for clinical impact. Competitive studies will integrate standardized approaches to sample collection, correlative assays, and advanced data analyses, ensuring that findings are reproducible, shareable and impactful across the field.
The Clinical Innovator provides up to USD 1.000.000 per trial. Funding may be used for investigator salary, patient costs, supplies, correlative assays, and data analyses. Payments are structured in milestones to ensure steady progress and trial completion.
Eligibility:
Candidates must be the Principal Investigator (PI) of the proposed study. CRI has no citizenship restrictions, and research supported by the award may be conducted at medical schools or research centers in the United States or abroad. CRI does not support research at for-profit institutions.
Deadline: 1 December 2025 for the protocol concept
